Second it yields exponential divergence so we get SD which is what many people expect for chaotic systems. Question 46 Write the favourable factors for the formation of ionic bond.
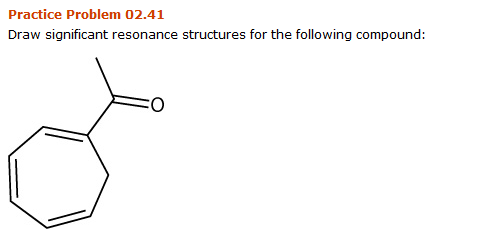
Solved Practice Problem 02 41 Draw Significant Resonance Chegg Com
Draw the products of this reaction draw the major product include stereochemistry if any.

. Close Log In. Naphthalene is stabilized by resonance. In the same Marvin editor draw the skeletal structures for both enantiomers that means you have to show wedgedash bonds formed For the following reaction label reactants and products as acidsbases and conjugate acidbases.
David Rawn in Organic Chemistry Study Guide 2015 166 The Williamson Ether synthesis. Write the resonance structures of a CH 3 COO- and Answer. NN-O NNO N-NO True or False.
Patients with diabetes are often required to check their blood glucose levels 310 timesday. Enter the email address you signed up with and well email you a. Question 47 Discuss the shape of the following molecules using the VSEPR model.
Earthquake-Resistant Design of Structures Second Edition Shashikant K. In a typical sophomore organic chemistry course theres about 14 functional groups that are key with another group. Log in with Facebook Log in with Google.
The Williamson ether synthesis is an S N 2 reaction in which an alkoxide ion is a nucleophile that displaces a halide ion from an alkyl halide to give an ether. Add octets to outer atoms. First it can be proven that Chaos_h implies Chaos_d.
Edu Mar 22 2017 A classic paper from a pioneer of physical. The skeletal formula also called line-angle formula or shorthand formula of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecules bonding and some details of its molecular geometryA skeletal formula shows the skeletal structure or skeleton of a molecule which is composed of the skeletal atoms that. Explore the world of molecule polarity defining covalent bonds electronegativity dipoles and dipole moments.
H2S SiCl4 BeF2 2 3 CO HCOOH Question 45 Define octet rule. Question 44 Draw the Lewis structures for the following molecules and ions. Common examples are alcohols amines carboxylic acids ketones and ethers.
Is prepared by the following reaction sequence. Add a multiple bond double bond to see if central atom can achieve an octet. Development of an in vivo glucose sensor that allows for.
Show the movement of electrons by curved arrows. A significant medical problem of the 21 st century is the growing incidence of diabetes mellitus a disease in which the body either does not produce its own insulin Type I or cells become insulin resistant Type II. TF N2O has a few resonance structures as shown below all lone pair electrons are purposeful left out.
Remember me on this computer. B CH 6 H 5 NH 2. The two structures on the left have one discrete benzene ring each but may also be viewed as 10-pi-electron annulenes having a bridging single bond.
The reaction occurs with inversion of configuration at chiral centers and can be limited by possible competing. It has 6 electrons. Functional groups are specific groupings of atoms within molecules that have their own characteristic properties regardless of the other atoms present in a molecule.
The smallest such hydrocarbon is naphthalene. Does central electron have octet. However it has a significant disadvantage in that it cannot be applied to invertible maps the kinds of maps characteristic of many systems exhibiting Hamiltonian chaos.
Write its significance and limitations. I have a whole series of articles where I discuss acidity trends and refer to electronegativity polarizability resonance adjacent electron withdrawing groups and even aromaticity. They differ in the position of atoms and hence are not resonance structures.
Add extra electrons 24-240 to central atom. The point of the current article is to mention that basicity is measured by pKa it is an equilibrium whereas nucleophilicity is measured by rate. Three canonical resonance contributors may be drawn and are displayed in the following diagram.
Which of the following pairs of structures do not constitute resonance structures. The central Boron now has an octet there would be three resonance Lewis structures However. Dipoles and dipole moments occur when electrons are shared unevenly by atoms.
All these three resonance structures are equally important contributors to the overall properties of N2O.

Solved Practice Problem 02 41 Draw Significant Resonance Chegg Com

Draw Significant Resonance Structures For The Following Compound Which Of This Is Are Most Significant Resonance Structures Study Com

Draw Significant Resonance Structures For The Following Compound Which Of This Is Are Most Significant Resonance Structures Study Com
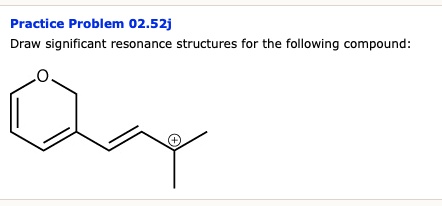
Solved Practice Problem 02 52j Draw Significant Resonance Structures For The Following Compound
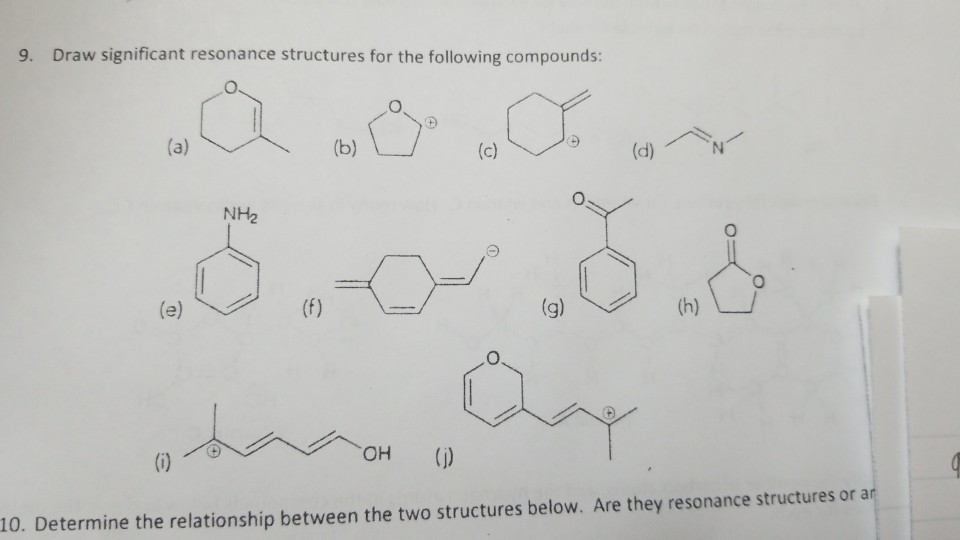
Solved 9 Draw Significant Resonance Structures For The Chegg Com

Solved Draw Significant Resonance Structures For The Chegg Com

Solved 2 41 Draw Significant Resonance Structures For The Chegg Com

1 Draw All Resonance Structures For The Following Compound Circle The Major Contributor To The Resonance Homeworklib
0 comments
Post a Comment